Research
CTOS
Biological characteristics of CTOS
CTOS preserves characteristics of original patient tumors in vitro as well as in CTOS-derived xenograft tumors. Followings are the examples of the biological characteristics of CTOS.
#1 Polarity switching of colorectal CTOS
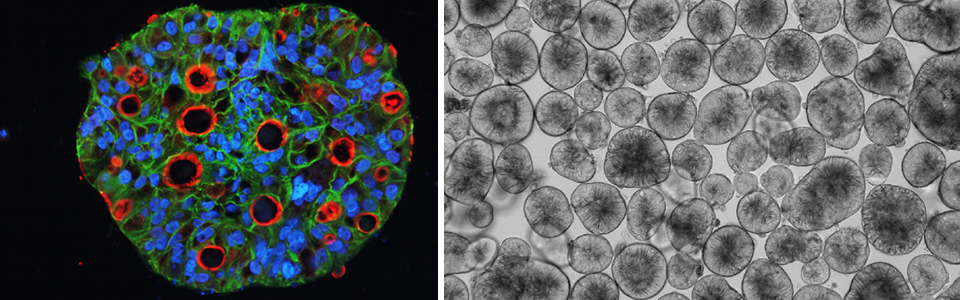
Epithelium is an interface between inside and outside of the body. The direction of the absorption and secretion is tightly regulated, which is based on the morphological asymmetry, apico-basal polarity. Outside is the apical membrane and inside is the basal membrane. It is often said that the polarity is ‘lost’ in cancer, but the truth is that the polarity is just ‘disorganized’. For example, most of the colorectal cancer (CRC) pathologically is differentiated adenocarcinoma, meaning that the polarity is retained in some extent. The polarity status is preserved in CTOS, but lost in most of the conventional cell lines even when cultured as spheroids.
In contrast to the necessity of extracellular matrix in organoid culture of the normal cells, CTOS can be cultured in suspension. In the floating CTOS, apical membrane is formed outside of the CTOS (apical-out). On the other hand, when CTOS is cultured in the extracellular matrix, apical membrane is formed surrounding small lumens inside CTOS (apical-in). We call the phenomenon as polarity switching (Figure 2-1) (1).
Microvessel invasion, defined by a presence of cancer-cell clusters inside the lumen of the blood or lymphatic vessels, is a pathological finding relevant to metastasis. Within the microvessel invasion of clinical CRC samples, the apical-in phenotype similar to the cultured CTOS in vitro was found, indicating that the polarity switching is a physiological phenomenon.
We found either the inhibitor of Src or dynamin inhibits the polarity switching, which occurs rapidly within a few hours responding to the presence/absence of extracellular matrix. When floating CTOS was pretreated by the dynamin inhibitors, and injected into the portal vein of the mice, liver metastasis was suppressed along with the retardation of polarity switching. This pretreatment also induced massive cell death in the cancer-cell clusters staying in the lumen of the intrahepatic portal vein.
Therefore, it is possible that the polarity switching occurs in the patient tumors and may be involved in the liver metastasis of the colorectal cancer.
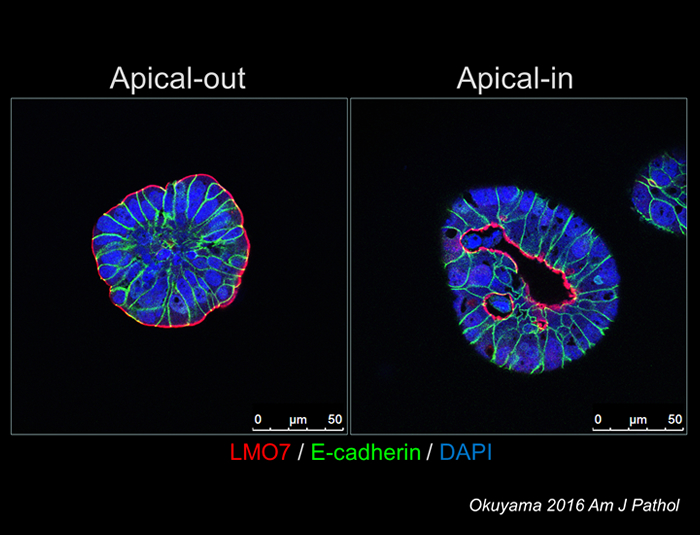
Figure 2-1: polarity switching in CTOS
#2 CTOS as an antigen for making monoclonal antibodies
CTOS preserves characteristics of original tumor, especially the polarity status in differentiated tumors. Using colorectal CTOS as an antigen, we generated monoclonal antibodies which recognize apical membrane outside of the floating CTOS (2). One of the antibodies suppressed attachment of CTOS to the collagen coated dish. The antigen was revealed to be glycan structures, which is mostly lost in the conventional cancer cell lines. Thus, CTOS might be useful for generating antibodies, especially against glycan structures which are lost in cancer cell lines. In addition, as the apical membrane is exposed to blood stream in the region of microvessel invasion, the antibodies might be applicable as a tumor marker or a therapeutic drug.
#3 Drug sensitivity assay
By virtue of easy preparation and stable culture of CTOS method, CTOS can be applied to drug or radiation sensitivity assay. It should be noted that CTOS method enables sensitivity assay with high-resolution quantification, such as drawing dose response curve, which has long been difficult to achieve using primary cultured cell. As a proof of concept, we evaluated the response to erlotinib, an EGFR tyrosine kinase inhibitor, of lung cancer CTOS derived from different patient tumors (3). The diverse responses were seen among the CTOSs; those with EGFR mutation responded well to erlotinib, which is compatible to clinical studies. In addition to evaluating growth or viability of the tumor cells, CTOS also allows analyzing intracellular signaling, which is a powerful tool for studying molecular targeting drug (Figure 2-2). Importantly, these in vitro results analyzed on CTOS highly correlated with the in vivo drug sensitivity evaluated with CTOS-derived xenografts. We also demonstrated that CTOS is applicable to the radiation sensitivity assay (4).
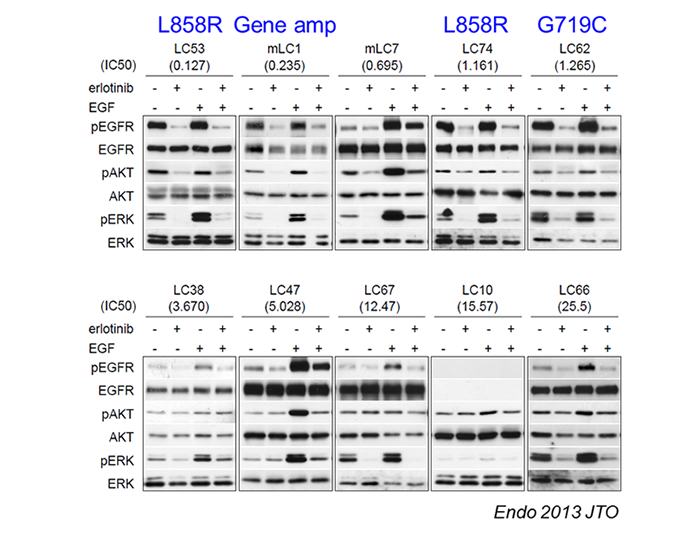
Figure 2-2: Evaluation of Intracellular signaling using CTOS
Highly efficient preparation of CTOS from tumors, especially from xenograft tumors, enables drug screening which requires large amount of cells. In addition, the hit drugs can be tested with multiple lines of CTOS from different patients to assess the inter-patient diversity of drug sensitivity.
We performed drug screening using endometrial cancer CTOS (5). About a hundred drugs were screened with two lines of CTOS, and a hit drug, YM155, was assessed dose response curve in 12 CTOS lines from different patients. Substantial diversity in the sensitivity was observed. Histological types as well as the type of cell death correlated with the sensitivity. Although the study was performed by manual picking of individual single CTOS under microscope, it is critical to develop an automated CTOS picking machine to screen thousands of drugs.
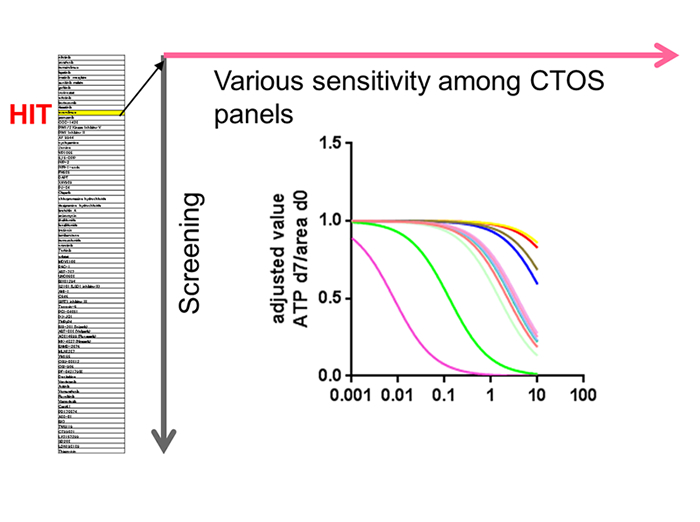
Figure 2-3: Strategy of drug screening with CTOS
#4 CTOS panel
We have been trying to apply CTOS methods to various cancers. CTOS can be prepared from colorectal, lung, urothelial, cervical, and endometrial cancer (1, 3-7). Preparation of CTOS from xenograft tumor is much easier than directly from patient tumor, and we have successfully prepared CTOS from patient-derived xenograft of gastric, pancreatic, breast, and prostate cancer. Some of the CTOS can be expanded by the cycle of xenograft formation and CTOS preparation followed by formation of next generation of xenograft. It enables large amount of CTOS to be freeze-stocked in early stages of the passage. We call it as CTOS line, using which we can perform reproducible experiments and accumulate information such as genome, drug sensitivity, gene expression, and so on. Currently, we have 45 lines of colorectal cancer and 34 of lung cancer.
Accumulating CTOS lines is useful especially for rare cancer, as it is practically impossible to develop therapeutic strategy with clinical trials as the number of the patient is too small. Small cell carcinoma of the cervix (SCCC) is found only 1% of the cervical cancer patients. Onset of the disease is relatively young, and the prognosis is poor. There is no cell line deposited in ATCC. We have been accumulating the lines of this rare cancer and established 9 lines so far (4). As the success rate of making CTOS lines from SCCC is quite high (100%), it should be tried in multiple institutes to realize the pre-clinical trial using CTOS lines.
- Okuyama H, Kondo J, Sato Y, Endo H, Nakajima A, Piulats JM, Tomita Y, Fujiwara T, Itoh Y, Mizoguchi A, Ohue M, Inoue M. Dynamic Change of Polarity in Primary Cultured Spheroids of Human Colorectal Adenocarcinoma and Its Role in Metastasis. Am J Pathol. 2016;186(4):899-911.
- Sato Y, Tateno H, Adachi J, Okuyama H, Endo H, Tomonaga T, Inoue M. Generation of a monoclonal antibody recognizing the CEACAM glycan structure and inhibiting adhesion using cancer tissue-originated spheroid as an antigen. Sci Rep. 2016;6:24823.
- Endo H, Okami J, Okuyama H, Kumagai T, Uchida J, Kondo J, Takehara T, Nishizawa Y, Imamura F, Higashiyama M, Inoue M. Spheroid culture of primary lung cancer cells with neuregulin 1/HER3 pathway activation. J Thorac Oncol. 2013;8(2):131-9.
- Nakajima A, Endo H, Okuyama H, Kiyohara Y, Kimura T, Kamiura S, Hiraoka M, Inoue M. Radiation sensitivity assay with a panel of patient-derived spheroids of small cell carcinoma of the cervix. Int J Cancer. 2015;136(12):2949-60.
- Kiyohara Y, Yoshino K, Kubota S, Okuyama H, Endo H, Ueda Y, Kimura T, Kimura T, Kamiura S, Inoue M. Drug screening and grouping by sensitivity with a panel of primary cultured cancer spheroids derived from endometrial cancer. Cancer Sci. 2016;107(4):452-60.
- Kondo J, Endo H, Okuyama H, Ishikawa O, Iishi H, Tsujii M, Ohue M, Inoue M. Retaining cell-cell contact enables preparation and culture of spheroids composed of pure primary cancer cells from colorectal cancer. Proc Natl Acad Sci U S A. 2011;108(15):6235-40.
- Okuyama H, Yoshida T, Endo H, Nakayama M, Nonomura N, Nishimura K, Inoue M. Involvement of heregulin/HER3 in the primary culture of human urothelial cancer. J Urol. 2013;190(1):302-10.
hypoxia
#1 Anti-angiogenesis treatment and tumor hypoxia
Escape of cancer cells from hypoxic microenvironment is driven by hypoxia response through activating the mechanisms related to invasion or metastasis. We and collaborators found that the tumors in a mouse model of pancreatic islet tumor (Rip-Tag mouse) showed remarkably invasive histological appearance when angiogenesis was impaired by genetic or pharmacological inhibition of VEGF signaling, although of the growth of the tumor was profoundly suppressed (Figure 3-1) (1). Inhibition of angiogenesis resulted in generating hypoxic area inside the tumor. We demonstrated that hypoxia response through Hif-1ホア was necessary for this invasive phenotype using conditional knockout of Hif-1ホア (2). Thus, the islet tumor cells might escape from hypoxic region due to anti-angiogenesis treatment, by invading into surrounding endocrine tissue where the tissue is well oxygenized.
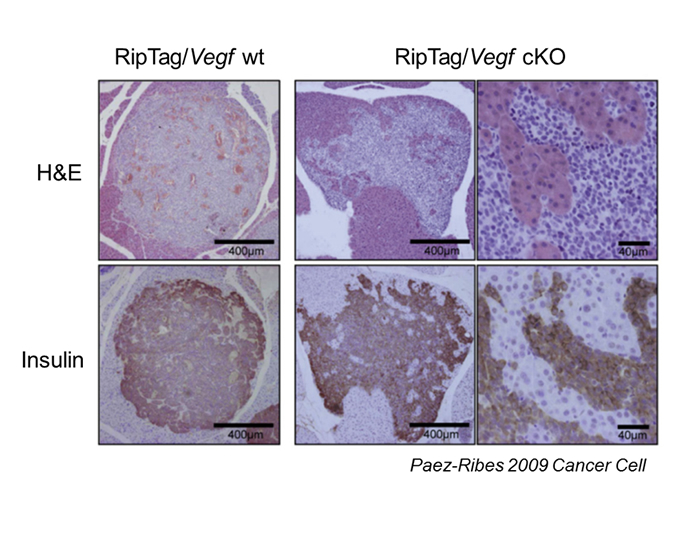
Figure 3-1: Invasive phenotype of islet cell tumors in RipTag; Vegf KO mouse
#2 Signaling of growth factor and oncogene in prolonged hypoxia
Oncogenic pathways including growth factor signaling are important for cancer growth. Meanwhile, in the tumors of either patient or xenograft, not all the cancer cells are actively proliferating, especially in hypoxic region. Little is known about the role of oncogenic pathways with the low proliferative activity, and the mechanism how the activity is suppressed.
IGF is known to be a survival and growth factor. Surprisingly, when Colo320, a colorectal cancer (CRC) cell line, under hypoxic conditions was treated with IGF, drastic apoptosis was induced (3). We found that robust ER stress was induced by IGF treatment. Inhibition of AKT or mTOR rescued the cell death. Thus, aberrant stimulation of IGF signaling in hypoxia rather triggered cell death.
MYC is an oncoprotein, which has a diverse function including activation of protein synthesis, cell cycle and mitochondrial biogenesis. MYC promotes energy consuming process in general, and activation of MYC increases both glucose and oxygen consumption. We found that MYC levels were low in hypoxic area of xenograft tumors (4). Protein levels of c-MYC in vitro strikingly decreased in CRC cell lines within few hours under hypoxic and glucose depleted conditions. The decrease was at least partly due to accelerated protein degradation. Forced suppression of c-MYC protein levels resulted in better survival of cancer cells under hypoxic and glucose depleted conditions (4). Thus, suppression of oncogenic pathway is beneficial for cancer cells to survive under deteriorated microenvironment.
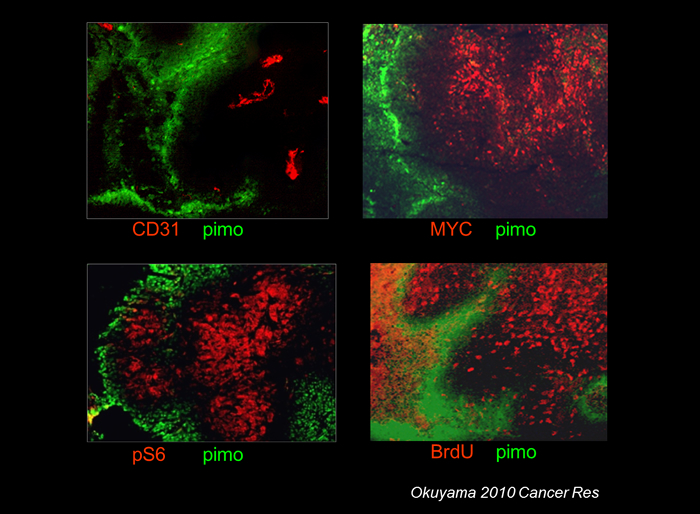
Figure 3-2: Decreased MYC levels in hypoxic region. Mouse xenograft of HCT116 CRC cell line.
#3 Tumor dormancy
Cancer is a proliferative disease, so that most of the cancer drugs have been developed to target proliferating cells. Historically, most of the in vitro studies on proliferation of the cancer cells have been made on established cancer cell lines. This means that large part of the nature of parental cancer cells, which are lost in the established cell lines, may have been overlooked.
Recently it has come to be realized that cancer cells exhibit remarkable plasticity depending on the microenvironment. In the tumors, not all the cancer cells are actively proliferating, especially in hypoxic region. Not only cell cycle but also other biological functions, such as metabolism are different from actively dividing cells. We hypothesized that what underlies the mechanism for the cancer cells to survive under deteriorated microenvironment is ‘cancer cell dormancy’, which is defined by three characteristics: 1) no growth, 2) no death, and 3) reversible. If cancer simply is a proliferative disease, one does not have to worry about dormant cells. But once the dormant cells can be reversible, they will be a reservoir of cancer cells, which might be a source of recurrence after treatment.
We have been trying to establish a model of tumor cell dormancy in vitro (5). Most of the established cancer cell lines in 2D culture conditions with serum containing medium cannot be dormant in hypoxia. They do not stop growing or die under hypoxic conditions. We found that a pancreatic cancer cell line, AsPC-1, exceptionally was able to be dormant; stopped growing, survived several weeks after prolonged culture in hypoxia, and regrew after being replaced in standard culture conditions. The dormant AsPC-1 cells showed less consumption of glucose and oxygen. Phosphorylation of AKT was repressed, which was necessary for being dormant. In contrast to the established cell lines, all of the CRC CTOS examined became dormant when they were cultured in hypoxia and growth factor depleted conditions. Therefore, a potential to be dormant in hypoxia is a common characteristics of cancer cells, which ability is mostly lost in conventional cell lines (5).
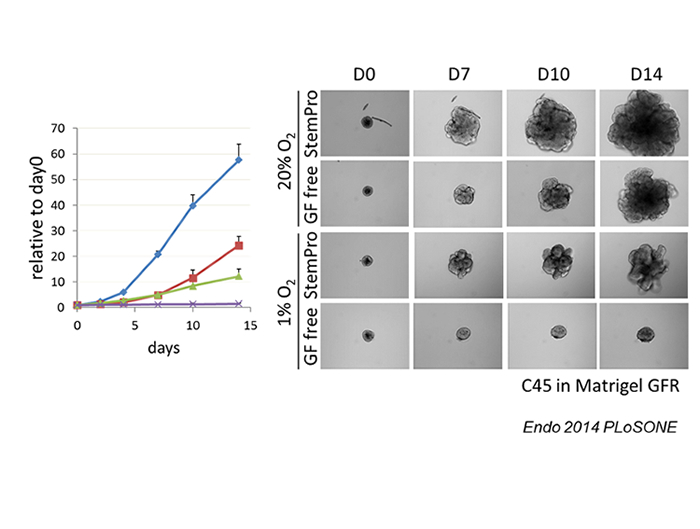
Figure: Dormancy of CRC CTOS
Inhibiting driver pathways is a principle of molecular targeting therapy. Taken together our findings about MYC and AKT (3, 4), driver pathways may be generally inactivated at the dormant status. If so, molecular targeting drug would not be effective for the dormant cells. With this hypothesis, we investigated EGFR mutant lung adenocarcinoma for which EGFR-TKI is used in clinic (6). EGFR mutant CTOS was able to be dormant under hypoxic conditions. Dormant CTOS was indeed resistant to EGFR-TKI. Constitutive phosphorylation of EGFR is maintained in dormant cells but downstream molecules such as AKT and ERK were inactivated. MIG6 is an endogenous inhibitor of EGFR signaling, which is induced by hypoxia. We found that MIG6 was induced in dormant status, and inhibited EGFR signaling by interfering dimer formation of EGFR with ERBB family receptors. Forced suppression of MIG6 levels by shRNA revealed that MIG6 was necessary for dormant status. Thus, EGFR mutant lung cancer cells turn down EGFR signaling to be dormant under hypoxic conditions, which may underlie the mechanisms for the EGFR-TKI resistance.
References
- Paez-Ribes M, Allen E, Hudock J, Takeda T, Okuyama H, Vinals F, Inoue M, Bergers G, Hanahan D, Casanovas O. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15(3):220-31.
- Takeda T, Okuyama H, Nishizawa Y, Tomita S, Inoue M. Hypoxia inducible factor-1alpha is necessary for invasive phenotype in Vegf-deleted islet cell tumors. Sci Rep. 2012;2:494.
- Endo H, Murata K, Mukai M, Ishikawa O, Inoue M. Activation of insulin-like growth factor signaling induces apoptotic cell death under prolonged hypoxia by enhancing endoplasmic reticulum stress response. Cancer Res. 2007;67(17):8095-103.
- Okuyama H, Endo H, Akashika T, Kato K, Inoue M. Downregulation of c-MYC protein levels contributes to cancer cell survival under dual deficiency of oxygen and glucose. Cancer Res. 2010;70(24):10213-23.
- Endo H, Okuyama H, Ohue M, Inoue M. Dormancy of Cancer Cells with Suppression of AKT Activity Contributes to Survival in Chronic Hypoxia. PLoS One. 2014;9(6):e98858.
-
Endo H, Okami J, Okuyama H, Nishizawa Y, Imamura F, Inoue M. The induction of MIG6 under hypoxic conditions is critical for dormancy in primary cultured lung cancer cells with activating EGFR mutations. Oncogene. 2016.